Alzheimer’s disease is an irreversible, progressive brain disorder that slowly destroys memory and thinking skills. And eventually, it will even undermine your ability to carry out the simplest of tasks. It is the most common cause of dementia among older adults (not counting the side effects of pharmaceutical drugs), and, in most people, symptoms first appear in the mid-60s. It is currently untreatable and irreversible, although there are five drugs of marginal value designed to temporarily help with memory and thinking problems. None of these drugs, though, treats the underlying cause of the disease or slows its progression. According to the CDC, 5.7 million Americans currently live with Alzheimer’s, a disease that kills more people than breast and prostate cancer combined. It is the sixth leading cause of death in the United States. But wait; it gets worse. The number of Americans with Alzheimer’s is expected to more than double to about 14 million over the next 40 years.
The exact causes of Alzheimer’s disease aren’t known, but the dominant theory is that it is related to problems with brain proteins that fail to function normally, disrupt the work of brain cells, and unleash a series of toxic events. In other words, neurons are damaged, lose connections to each other, and eventually die. Specifically, the two hallmark pathologies required for a diagnosis of Alzheimer’s disease are the extracellular plaque deposits of the β-amyloid peptide (Aβ) and the flame-shaped neurofibrillary tangles of the microtubule binding protein tau:1 M. Paul Murphy and Harry LeVine, III. “Alzheimer’s Disease and the ß-Amyloid Peptide.” J Alzheimers Dis. 2010 Jan; 19(1): 311. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2813509/
 Beta-amyloid peptides (Aβ) are leftover fragments of arger proteins. When these fragments cluster together, they have a toxic effect on neurons and disrupt cell-to-cell communication. Over time, these clusters form larger deposits called amyloid plaques, which also include other cellular debris. It should be noted that roughly 30% of people who have brains “chock-full” of ß Aβ (at autopsy) never showed any signs of dementia before they died, which is where tau proteins come into play.
Beta-amyloid peptides (Aβ) are leftover fragments of arger proteins. When these fragments cluster together, they have a toxic effect on neurons and disrupt cell-to-cell communication. Over time, these clusters form larger deposits called amyloid plaques, which also include other cellular debris. It should be noted that roughly 30% of people who have brains “chock-full” of ß Aβ (at autopsy) never showed any signs of dementia before they died, which is where tau proteins come into play.- Tau proteins play a part in a neuron’s internal support and transport system to carry nutrients and other essential materials into the neuron–and waste material out. In Alzheimer’s disease, tau proteins change shape, misfold, and organize themselves into structures called neurofibrillary tangles. These tangles disrupt the transport system and are toxic to cells. Some researchers suspect that it is the combined insults of Aβ and tau that drive the often-dramatic decline seen in Alzheimer’s. Although the brain may be able to compensate for the deficits caused by ß-amyloid, once tau starts to spread, that pushes your brain over the edge.
Alzheimer’s disease is characterized clinically by a progressive and gradual decline in cognitive function driven by the build-up of Aβ plaque and tau proteins. The damage most often starts in the hippocampus, the region of the brain that controls memory, but the process begins years before the first symptoms ever appear. The loss of neurons spreads in a somewhat predictable pattern to other regions of the brains. By the late stage of the disease, the brain has shrunk significantly.
Scientists believe that, for most people, the changes in brain proteins is caused by a combination of genetic, lifestyle, and environmental factors that affect the brain over time. (In less than 1% of cases, Alzheimer’s is the result of specific genetic changes that virtually guarantee a person will develop the disease regardless of lifestyle. These rare occurrences usually result in the onset of the disease in middle age.
To summarize, most current Alzheimer’s research is focused on Aβ and tau.
A Different Theory
 Back in 2016, Berislav Zlokovic, the director of the Zilkha Neurogenetic Institute,2 http://keck.usc.edu/zilkha/ received a series of grants to explore his theory that leaky blood vessels in the brain might be a prime driver of Alzheimer’s and that fixing those leaks might prevent the disease. In 2019, the results of that study were finally published, and they are interesting to say the least.
Back in 2016, Berislav Zlokovic, the director of the Zilkha Neurogenetic Institute,2 http://keck.usc.edu/zilkha/ received a series of grants to explore his theory that leaky blood vessels in the brain might be a prime driver of Alzheimer’s and that fixing those leaks might prevent the disease. In 2019, the results of that study were finally published, and they are interesting to say the least.
As Dr. Zlokovic, who is also chair and professor of the physiology and biophysics department at the Keck School of Medicine of USC, explains, “The brain contains 400 miles of blood vessels that can stretch from Los Angeles to San Francisco. If there is a leak along this vascular tube and the pothole is, for example, near the hippocampus — the center of learning and memory — that can contribute to the development of dementia and Alzheimer’s disease. We can delay the onset or slow the progression of Alzheimer’s if we are able to fix leaky capillaries when they first start, some 10 to 15 years before Alzheimer’s symptoms even surface.”
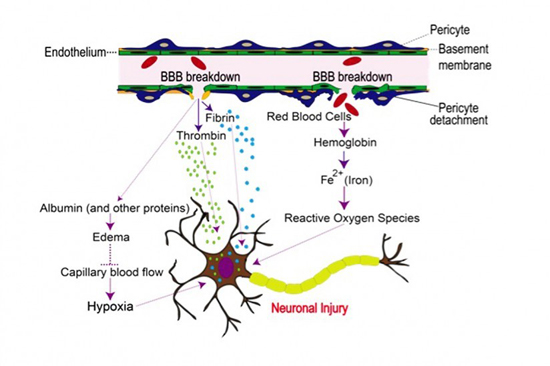
According to Zlokovic’s theory, when the blood-brain barrier breaks down, the leak along this vascular tube allows many blood-derived toxic products, cells, and pathogens to enter the brain and damage circuits involved in memory and learning.
Most people misunderstand the nature of the blood-blood barrier (BBB). They think of it is some protective covering that sits around the brain preventing anything bad from entering. That’s not quite correct. In fact, it’s the exact opposite. The BBB doesn’t “cover” the brain; it doesn’t sit “around” it. In fact, it sits inside the brain, integral to every inch of those 400 miles of blood vessels that reach into the smallest nook and cranny, providing oxygen and nutrients to every single cell in the brain. The blood-brain barrier is formed by the endothelial cells that line all 400 miles of cerebral microvasculature that feed the brain. Its role is to protect the brain from fluctuations in plasma composition, and from any circulating agents such as toxins, neurotransmitters, and xenobiotics capable of disturbing neural function.
Again, according to Zlokovic’s theory, “Blood-brain barrier’s leaks allow many blood-derived toxic products, cells, and pathogens to enter the brain and directly damage brain circuits involved in memory and learning. Additionally, bad proteins that normally are ejected from the brain, such as beta-amyloid, have to struggle against the flow of traffic when trying to exit the brain. This situation can eventually lead to ‘a second hit’: Beta-amyloids and tau tangles remain in the brain and cause damage that become evident years later.”
This is a key point in Zlokovic’s theory. Beta-amyloids and tau tangles still play an important role in the onset of Alzheimer’s, but it is now a supporting role–secondary to the leakage of capillaries in the brain.
Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction
The results of the study were published in the January 14, 2019 issue of Nature Medicine.3 Daniel A. Nation, Melanie D. Sweeney, Axel Montagne, Berislav V. Zlokovic, et al. “Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction.” Nature Medicine volume 25, pages270–276 (2019). http://www.nature.com/articles/s41591-018-0297-y In short, the study found that individuals with early cognitive dysfunction develop brain capillary damage and blood-brain barrier breakdown in the hippocampus irrespective of Aβ and/or tau biomarker changes. These findings support Zlokovic’s theory that BBB breakdown is an early biomarker of human cognitive dysfunction independent of Aβ and tau. Or in laymen’s terms: Leaky capillaries in the brain likely are predictive of the onset of Alzheimer’s disease as they signal cognitive impairment before hallmark toxic proteins appear.
The 161 participants in the study had their memory and thinking ability assessed through a series of tasks and tests, resulting in measures of cognitive function and a “clinical dementia rating score.” Individuals diagnosed with disorders that might on their own account for cognitive impairment were excluded. The researchers used neuroimaging and cerebral spinal fluid analysis to measure the permeability, or leakiness, of capillaries serving the brain’s hippocampus, and found a strong correlation between impairment and leakage.
“The results were really kind of eye-opening,” said first author Daniel Nation, an assistant professor of psychology at the USC Dornsife College of Letters, Arts and Sciences. “It didn’t matter whether people had amyloid or tau pathology; they still had cognitive impairment.”
The researchers cautioned that their findings represent a snapshot in time. In future studies, they hope to get a better sense of how soon cognitive problems occur after blood vessel damage appears. Zlokovic said it’s unlikely that scientists will soon abandon amyloid and tau as Alzheimer’s biomarkers, “but we should be adding some vascular biomarkers to our toolkit.”
So, What Does It Mean?
As Dr. Zlokovic said, “The fact that we’re seeing the blood vessels leaking, independent of tau and independent of amyloid, when people have cognitive impairment on a mild level, suggests it could be a totally separate process or a very early process. That was surprising that this blood-brain barrier breakdown is occurring independently.”
It should be noted that studies have demonstrated that the brain’s endothelial cells differ from the endothelial cells in capillaries of other body organs in two important ways.4 Brightman MW. “Morphology of blood-brain interfaces.” Exp Eye Res. 1977;25 Suppl:1-25. http://www.ncbi.nlm.nih.gov/pubmed/73470
- There are no detectable trans-endothelial pathways. In other words, there’s no pathway “through” the endothelial cells.
- And there’s no way around the cells, as continuous tight junctions are present between the endothelial cells, which prevents transcapillary movement of polar molecules varying in size from proteins to ions.
As a result of these special anatomical features, the endothelial cells in the brain’s cardiovascular system provide a continuous cellular barrier between the blood and the interstitial fluid. This is the blood-brain barrier. The ability of the BBB to maintain tight junctions between cells is primarily determined by the junctional complexes between the cerebral endothelial cells.5 Svetlana M Stamatovic, Allison M Johnson, Richard F Keep, and Anuska V Andjelkovic. “Junctional proteins of the blood-brain barrier: New insights into function and dysfunction.” Tissue Barriers. 2016 Jan-Mar; 4(1): e1154641. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4836471/ Although there are strong similarities to epithelial cells in other parts of the body, brain endothelial junction complexes have a specific structural organization and a unique protein expression pattern. Specifically, the brain endothelial junction complex is a highly elaborate structure of protein strands arranged as a series of multiple barriers that pull the cells tightly together and completely seal the clefts between the brain’s endothelial cells. Any damage to the proteins in these junction complexes can directly lead to a widening of the gaps between cells, compromising the BBB, and resulting in blood leakage.
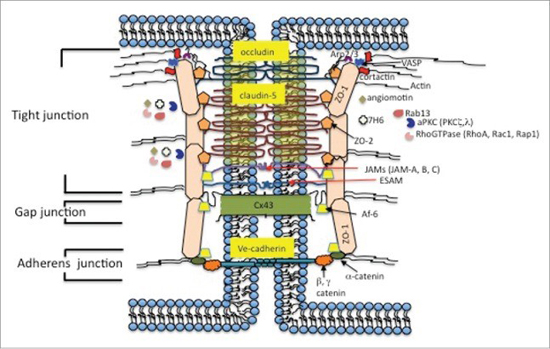
In healthy brains, the cells that make up blood vessels fit so tightly together that they keep stray cells, pathogens, metals, and other unhealthy substances from reaching brain tissue–not to mention any blood from leaking into the brain. In some brains, especially aging brains, the protein junctions become degraded, seams between cells loosen, and the blood vessels become permeable.
“If the blood-brain barrier is not working properly, then there is the potential for damage,” said co-author of the study Arthur Toga, director of the USC Mark and Mary Stevens Neuroimaging and Informatics Institute at the Keck School of Medicine. “It suggests the vessels aren’t properly providing the nutrients and blood flow that the neurons need. And you have the possibility of toxic proteins getting in.”
(Graphic/The Zlokovic Lab)
Does this Change What I Should Do to Protect Myself from Alzheimer’s?
Not really. But it does make it clearer why you should do some of the things already recommended.
- L-carnosine pushes off cell senescence (including that of the brain’s endothelial cells) and protects tissue and organs comprised of proteins. It also prevents the build-up of Aβ plaque in the brain. For these reasons, supplementing with an L-carnosine based formula has always been recommended as nutritional support for brain tissue and as a defense against the onset of Alzheimer’s. Now, based on the new study, there is yet another reason for supplementing with carnosine since it likely will help protect the proteins that hold the epithelial cells of the brain tightly together and, thus, prevent leakage from occurring–being able to reach these proteins as it has the inherent ability to cross the blood-brain barrier.6 Jin CL, Yang LX, Wu XH, Li Q, Ding MP, Fan YY, Zhang WP, Luo JH, Chen Z. “Effects of carnosine on amygdaloid-kindled seizures in Sprague-Dawley rats.” Neuroscience. 2005; 135: 939–947. http://www.sciencedirect.com/science/article/abs/pii/S0306452205007293?via%3Dihub
- It has been theorized that the very factors that allow the brains vasculature to conduct its vital functions predispose it to oxidative stress.7 Linnea R Freeman and Jeffrey N Keller. “Oxidative Stress and Cerebral Endothelial Cells: Regulation of the Blood-Brain-Barrier and Antioxidant Based Interventions.” Biochim Biophys Acta. 2012 May; 1822(5): 822–829. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3412391/ In other words, the endothelial cells that comprise the blood-brain-barrier are highly susceptible to free radical damage. Thus, supplementing with a full-spectrum antioxidant formula (especially one containing SOD, OPCs, selenium, green tea extract, quercetin, resveratrol, and NAC) makes sense to nutritionally support those cells in preventing and reversing oxidative damage and thereby reducing the risk of leakage.
- And make sure your diet includes an adequate supply of the following nutrients to optimize the health of the epithelial tissue in your brain’s microvasculature.
- Vitamin C
- Full complex natural vitamin E, with tocotrienols
- Hesperidin
- Arginine
- Omega-3 fatty acids
- Zinc
- Silica
Look, some risks are unavoidable in life. But even when unavoidable, you can still sometimes tweak the odds. Unless you are one of the tiny percentage who has a genetic predisposition to early-onset Alzheimer’s, based on what we now know about the disease, it certainly seems that you can move the odds in your favor in terms of coming down with the disease–or at least pushing its onset far enough out that you’ll never notice any symptoms in your lifetime. And as anyone who has been to Vegas can tell you: when playing against the casino of life, that’s a sweet deal.

References
| ↑1 | M. Paul Murphy and Harry LeVine, III. “Alzheimer’s Disease and the ß-Amyloid Peptide.” J Alzheimers Dis. 2010 Jan; 19(1): 311. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2813509/ |
|---|---|
| ↑2 | http://keck.usc.edu/zilkha/ |
| ↑3 | Daniel A. Nation, Melanie D. Sweeney, Axel Montagne, Berislav V. Zlokovic, et al. “Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction.” Nature Medicine volume 25, pages270–276 (2019). http://www.nature.com/articles/s41591-018-0297-y |
| ↑4 | Brightman MW. “Morphology of blood-brain interfaces.” Exp Eye Res. 1977;25 Suppl:1-25. http://www.ncbi.nlm.nih.gov/pubmed/73470 |
| ↑5 | Svetlana M Stamatovic, Allison M Johnson, Richard F Keep, and Anuska V Andjelkovic. “Junctional proteins of the blood-brain barrier: New insights into function and dysfunction.” Tissue Barriers. 2016 Jan-Mar; 4(1): e1154641. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4836471/ |
| ↑6 | Jin CL, Yang LX, Wu XH, Li Q, Ding MP, Fan YY, Zhang WP, Luo JH, Chen Z. “Effects of carnosine on amygdaloid-kindled seizures in Sprague-Dawley rats.” Neuroscience. 2005; 135: 939–947. http://www.sciencedirect.com/science/article/abs/pii/S0306452205007293?via%3Dihub |
| ↑7 | Linnea R Freeman and Jeffrey N Keller. “Oxidative Stress and Cerebral Endothelial Cells: Regulation of the Blood-Brain-Barrier and Antioxidant Based Interventions.” Biochim Biophys Acta. 2012 May; 1822(5): 822–829. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3412391/ |